We get it. You're used to your current EDC system, and it seems too much time and effort are required to switch. Yet, our clients have found that upgrading to Viedoc improves trial efficiency from day one.

One of the most common frustrations in clinical research today can be traced to outdated data management solutions with different applications that don’t communicate with each other. Too much time is wasted manually importing and exporting, putting your clinical trial data at risk. Viedoc offers one scalable, insightful EDC system that securely transfers information with seamless integration between each application to save you time—no more lags.


Stop waiting for your vendor to fix your trial. Become independent with us and in-house your clinical trial setup—so you can cut study timelines from the very beginning. With Viedoc, you spend less time responding to technical problems and more time focusing on your study. Viedoc provides seven applications in one unified platform, giving you all the tools you need to design, manage, and configure brilliant studies every time.


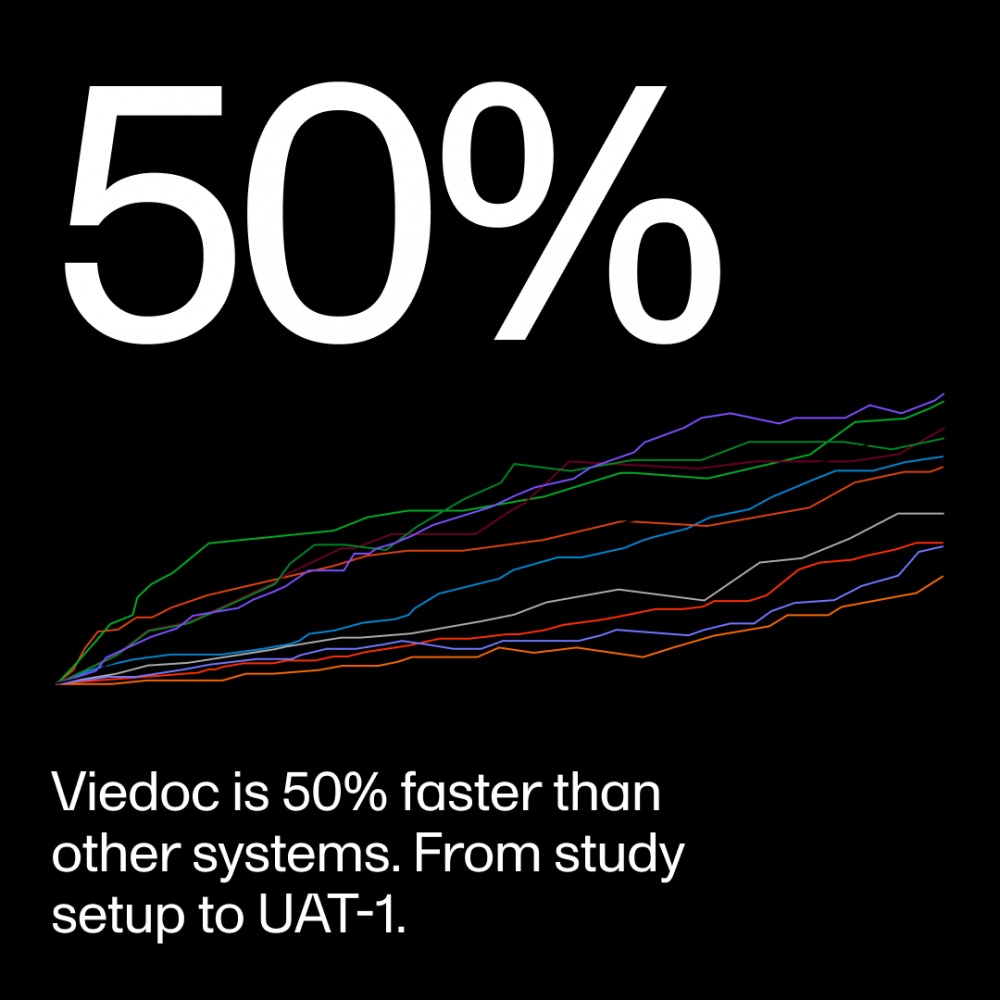
Viedoc is built to support all the traditional pathways required to build sophisticated studies. Our intuitive interface is second to none, giving you faster setups in a cost-effective solution that is unmatched in the EDC world today. Up to 50% faster from study setup to User Acceptance Testing (UAT-1). Who can beat that?
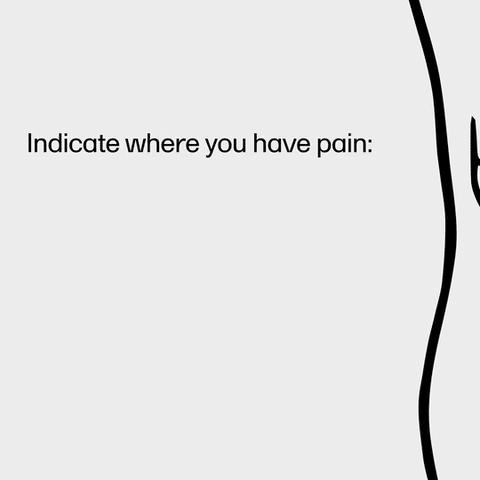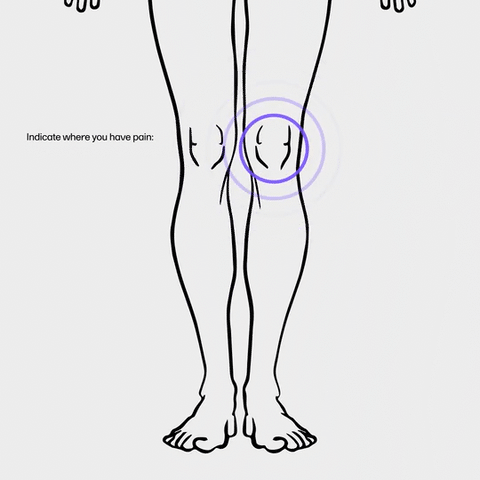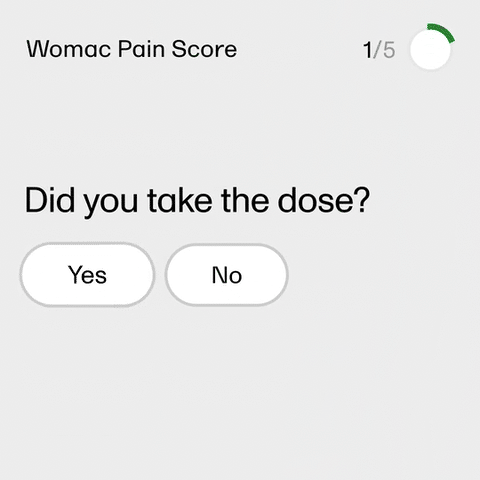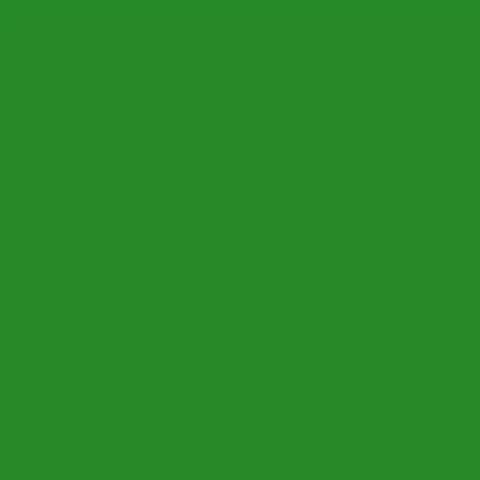
It’s the 2020s, and everyone is on the go, so platforms should be accessible anytime, anywhere. Viedoc created a unique blend of functionality and design perfectly adapted to any device, whether it’s a tablet, phone, desktop, or laptop. We've added built-in modern functions, such as auto-reminders via SMS or email, and interactive, customizable scales. Video calls, smooth image capture, and file uploads are quick and easy for everyone with our telemedicine solution, Viedoc Connect, and our engaging ePRO/eCOA, Viedoc Me.
Essentially, sites need to get more done with fewer resources—and most sites agree that technology can make that happen. Clinical research professionals built Viedoc with industry needs and wants in mind. Your team can be trained on Viedoc in 8–16 hours max and freshen up their knowledge on the fly with our fully integrated eLearning solution, which offers video tutorials and role-based user guides. Configure user roles and set permissions in Viedoc Designer, then use Viedoc Admin to assign and modify user roles and delegate activities to different site managers. With Viedoc, your clinical team works smarter, not harder.

With support for certified Viedoc Designers included, you can create your own designs or get inspiration from one of ours—we provide you with constantly improved and ready-made templates in our CDISC CDASH library to give you a head start in minutes. You can even build your next study during the certification process, and, once certified, you can train site and monitoring staff on your own. Adapt Viedoc across studies and scale to each phase. Manage and configure settings from the get-go without worry—your data is always backed up, 24/7.


Unlimited sandbox access allows you to test and deploy new study designs in your own time and allows designers to learn more about the functionalities in Viedoc Designer without stress. Take advantage of the industry’s fastest certification program with gold-standard training.
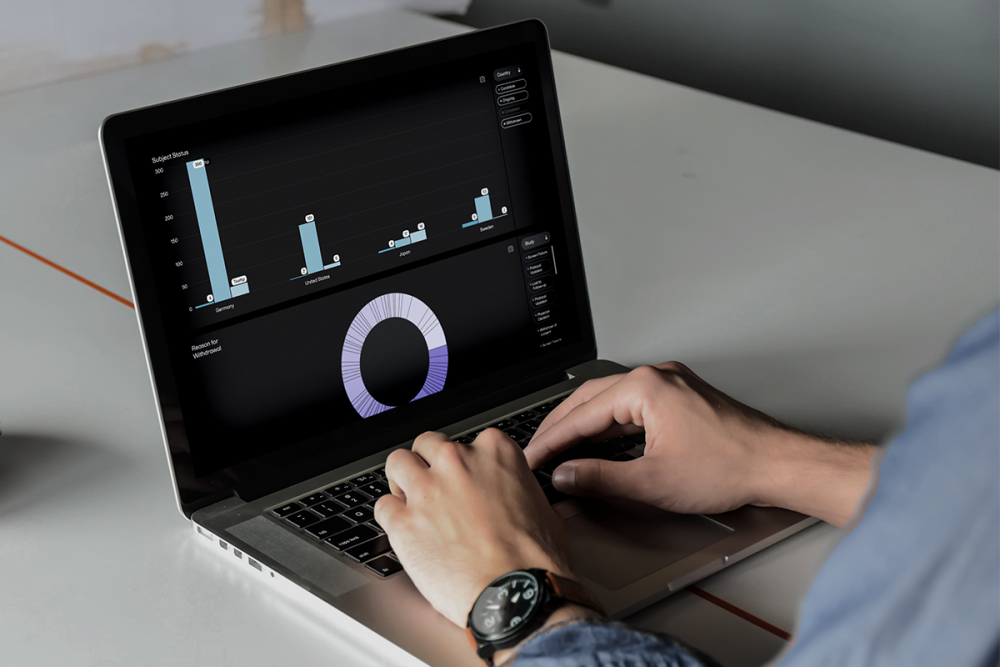
With a single database, you can conduct cross-application analysis and access real-time reports for quicker, deeper insights with ease. Work uninterrupted between applications, knowing trial data is continuously updated and always available across the system with auto-backup and 24/7 protection. Adjust as you go without any data loss or interruptions—improving data quality throughout your study.
With Viedoc, you’re always compliant and secure—we hold ISO 27001 certification, the highest international standard for information security. We help you prepare for inspections quickly with statements of compliance to fulfill 21 CFR part 11 in downloadable form, a Viedoc Inspection Readiness Packet (VIRP), and detailed audit trails for your records. We also ensure backward compatibility for all new releases.

Prepare for elevated reports. Viedoc Reports gives your data manager easy access to deeper insights with one reporting tool designed to make data beautiful. Features like Key Performance Metrics, a customizable dashboard, unlimited custom reports, and more give you a flexible view of your data so you can optimize resources and assess site performance.

In this field, time is expensive—and improving trial efficiency is the best way to cut time and costs. But much more important than that, trial timelines affect how quickly patients can access new treatments. We want to empower your organization and give you back time—your most valuable resource. At Viedoc, we pride ourselves on having created a patient-centric solution—one simple platform that everyone can easily access, makes our clients money, reduces costs, and helps bring treatments to patients faster.
Our eClinical solution is easy to adopt, validate, and implement, and we’re with you every step of the way. We give you a premium platform at an affordable cost that has the core solution and optional add-ons you need for any type of trial—and we continue to develop our technology with every release. That’s right—when you choose Viedoc, you choose a future-proof platform. Curious to see if Viedoc can be a good fit for your organization? Contact us and allow us to help you decide.

• One scalable system with integrations saves you time and hassle
• Cut timelines—Viedoc is 50% faster than other systems, from study setup to UAT-1—without compromising quality or data integrity
• Be independent by 'in-housing' your trial setup with lightning-fast study builds
• Improve patient retention and recruitment with an intuitive interface
• De-stress site staff with easy-to-use tools and user roles
• Create your unique design with ready-made templates or reuse your own
• Unlimited sandbox access to learn and test designs at any time
• A reporting tool that gives you deeper insights on a beautiful interface
• One single database with auto-backup and 24/7 protection
• ISO 27001 certification, 100% compliance with industry regulations
• Cross-application analysis and real-time reports for quicker, deeper insights


"The system is incredibly robust and is very user friendly, both to sponsor level satff, as well as site staff who remotely enter data."