It’s no secret that the modern patient demands innovative technology—one that’s easy to use, without steep learning curves or outdated approaches. That’s why we built the most up-to-date, engaging ePRO/eCOA solution on the market—Viedoc Me. Designed to ensure the smoothest and simplest data collection process yet for you and your patients, directly from the source. Everyone can easily connect to Viedoc via their device anywhere, from Canada to Cambodia. See the symptoms and side effects patients are experiencing as they report them, so you can intervene at a moment’s notice, and set languages for translation to get local and speak everyone’s language with ease.

Viedoc Connect is our versatile and responsive telemedicine solution, which allows you to interact with subjects via Viedoc Me the same way you would on-site, but remotely. Easily connect to your patients through secure video calls and run activities remotely. Facilitate the eConsent process, run pre-screening and recruitment activities, and conduct follow-up visits when on-site visits aren’t possible. Do your job seamlessly, all with the click of a button, without switching apps.

Both patients and site staff benefit from decentralized research. Did you know that 70% of potential study participants live more than two hours away from trial sites? Decentralizing your research enables you to diversify your recruitment process and reach more people. Save time and money for both you and your study participants, and provide a better efficacy and safety profile at the end. With Viedoc’s customizable solution, you can design, manage, and execute your remote clinical studies all within one scalable system. We’re the foundation with boundless capabilities.
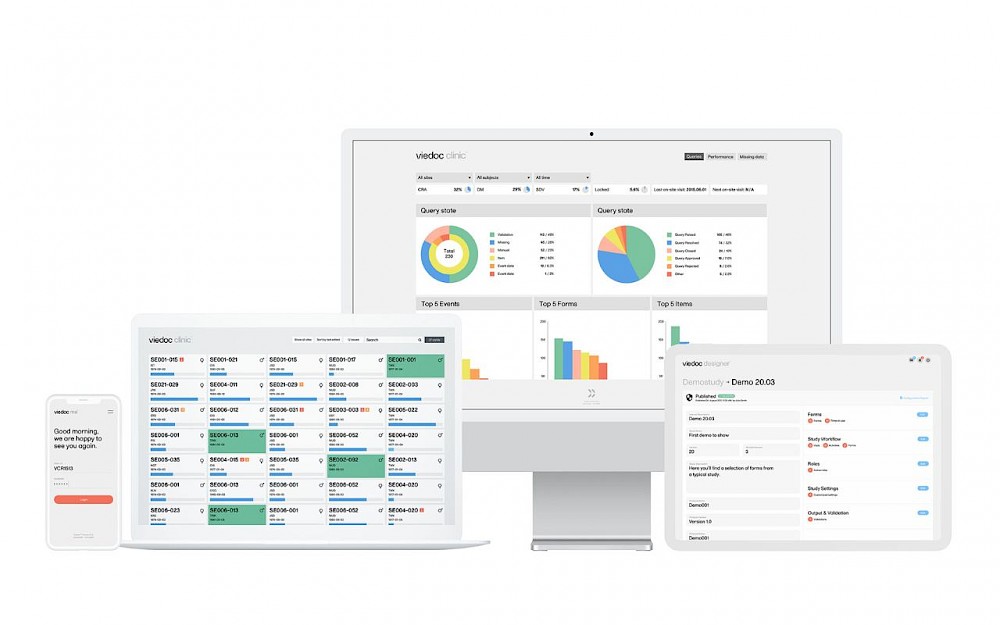
Data collection
Features for collection, viewing and reviewing of CRF data in an ICH GCP compliant manner, including capture of binary data (images / documents)
Sign data on form, visit or patient level Link data between forms (e.g. AE and CM)
Laboratory reference values with time, location and factor scope
Study building
Drag-and-drop form design with more than 18 different item types to choose from
Form preview allowing the designer to verify layout, conditions and checks directly onscreen
Automatic creation of blank and annotated CRFs
CDISC CDASH form library with over 20 ready-to-use forms
Ready-to-use study templates in CDISC ODM XML format
Form translator for managing multiple study languages
Best-in-class support for complex study designs / requirements
Study management
Role delegation service
Study level database lock feature
Study-recreation from a previous snapshot (CDISC ODM)
Unique and fully self-service study decommissioning feature including status reports and archiving recommendations
Documentation and certification management
Assignment of study designs
Study settings
Study license management
API management
TMF management
RTSM management
Reference data management
Medical coding dictionary management
User management
Site creation, with code, time zone, type (production / training), recruitment metrics
User management, with invites, resets, and removals
An integrated and configurable ePRO / eCOA feature according to the BYOD model
Supports custom workflows and complex decision trees Televisits
Reminders in local language via SMS and email Customizable VAS
File upload
Drawing pad with body map, signature line, or blank background
Support for unscheduled data entries
40 languages including righttoleft typing
Library of validated ePRO / eCOA forms
Integration with smartwatches, thermometers, scales, and more
Coming soon. Our eConsent process is smooth and reliable. Once combined, our range of products deliver an enhanced user experience with maximum flexibility for both the site and the participant. Remotely share essential information with participants and assess their understanding, expedite participant identification, and retrieve signatures.
Secure peer to peer video calls
Screen sharing
No installs or downloads
Easy access
Join in one click
Audit log tracking date, time, duration and participants
24 / 7 output to Excel, CSV, SAS, PDF / A (compliant to FDA submission, eCTD) and CDISC ODM formats
Scheduled exports
Online data preview and chart visualization
API for import and export of data in CDISC ODM
Real-time metrics on data quality and performance

"What I like best about Viedoc is their approachable support and "can do" attitude. Their service has always been personal and reliable."