
We sat down with Yusuke Nakazawa, Vice President of Business Development in Japan, to discuss what sets Viedoc PMS apart and to identify what features are essential to a PMS study.
- This is the most important feature. Usually, when data is entered into Viedoc Clinic, the sponsor can see this immediately. But in this integration, the sponsor cannot see the data until it is sent to them.
A fundamental requirement for a Japanese PMS study is to send and receive data on request. In Viedoc PMS, the Kaifu functionality, also known as Send/Receive, is a standard feature. The investigator using Viedoc Clinic can choose when to share data with the sponsor, and the sponsor or CRO can also decide when to receive the data.
- Most EDC systems don’t have this feature. In Japanese PMS studies, they don’t exchange each form but instead, put those forms into a folder and then send out those folders out to sponsors.
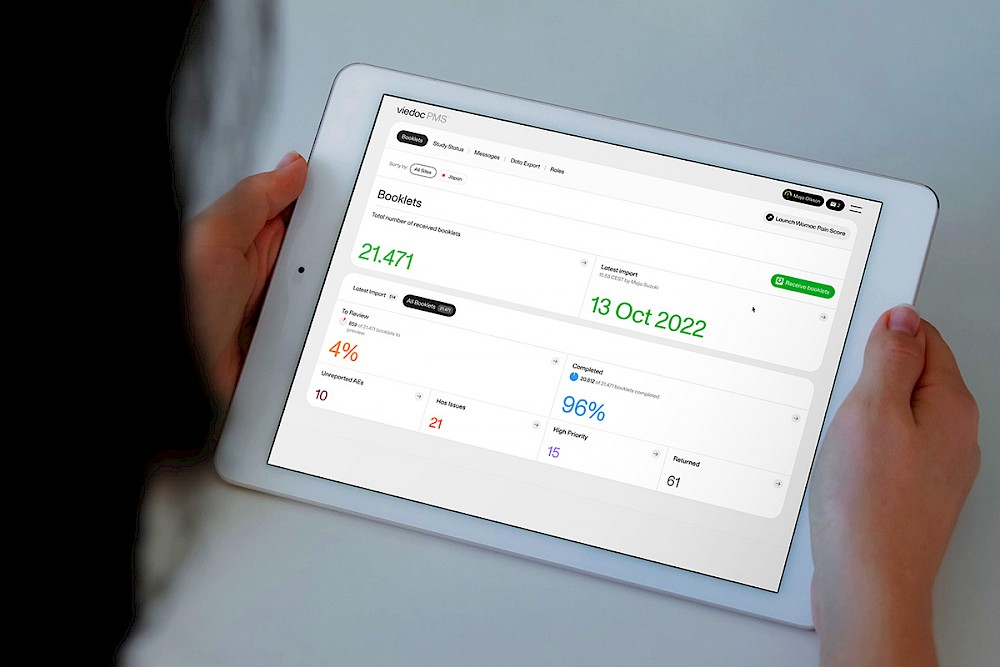
Booklets mirror the data collection and review process to match the workflow of a Japanese PMS study. Essentially, a booklet is a compilation of the data collected during a fixed period of time rather than at a particular visit.
- The team cannot send out those booklets until all forms are completed, but if they find something in error on the Clinic side, they need to report those errors immediately.
This feature supports error identification and reporting. In the Japanese PMS studies, as soon as the sponsor discovers data on adverse events (AEs) such as side effects, they are required to report the data to the authorities within 24 hours from the moment they discover the data. Partial submission allows AEs to be partially sent to the sponsor, even if the entire booklet is not yet ready for submission.


Viedoc 4.72: In the API configuration window, we now have a new option (Data Controller) for the Web API client configuration, and this allows the API manager to select Clinic side or Sponsor side to further define the scope of the API client.
Viedoc 4.71: Two new options for the Study settings in Viedoc Admin are available. These are set to either mandatory or optional—Require Contract for booklet submission and Require Responsible Investigator for booklet submission. Read more here.

The features essential to PMS studies are not available in a traditional EDC system—but they are available in Viedoc. To bring our innovative and seamless platform to Japan and fulfill the market’s requirements, Viedoc PMS gives our clients the same robust and flexible features available in Viedoc Clinic and total control of every type of trial run in Japan.
Between our office in Tokyo and Viedoc PMS, our team is fully dedicated to fulfilling the needs of the Japanese market—because we want our clients to be globally capable.
Contact us to learn how we can fulfill your unique trial requirements.

"Viedoc's UI always amazes me. Clean, simple, convenient and user friendly."