
Viedoc addresses one of the most common frustrations in clinical research: outdated data management solutions. By offering a single, scalable, and insightful EDC system, users can seamlessly transfer information between applications, saving valuable time and minimizing the risk of errors. As one reviewer mentioned, "The seamless integration between different applications is a game-changer."

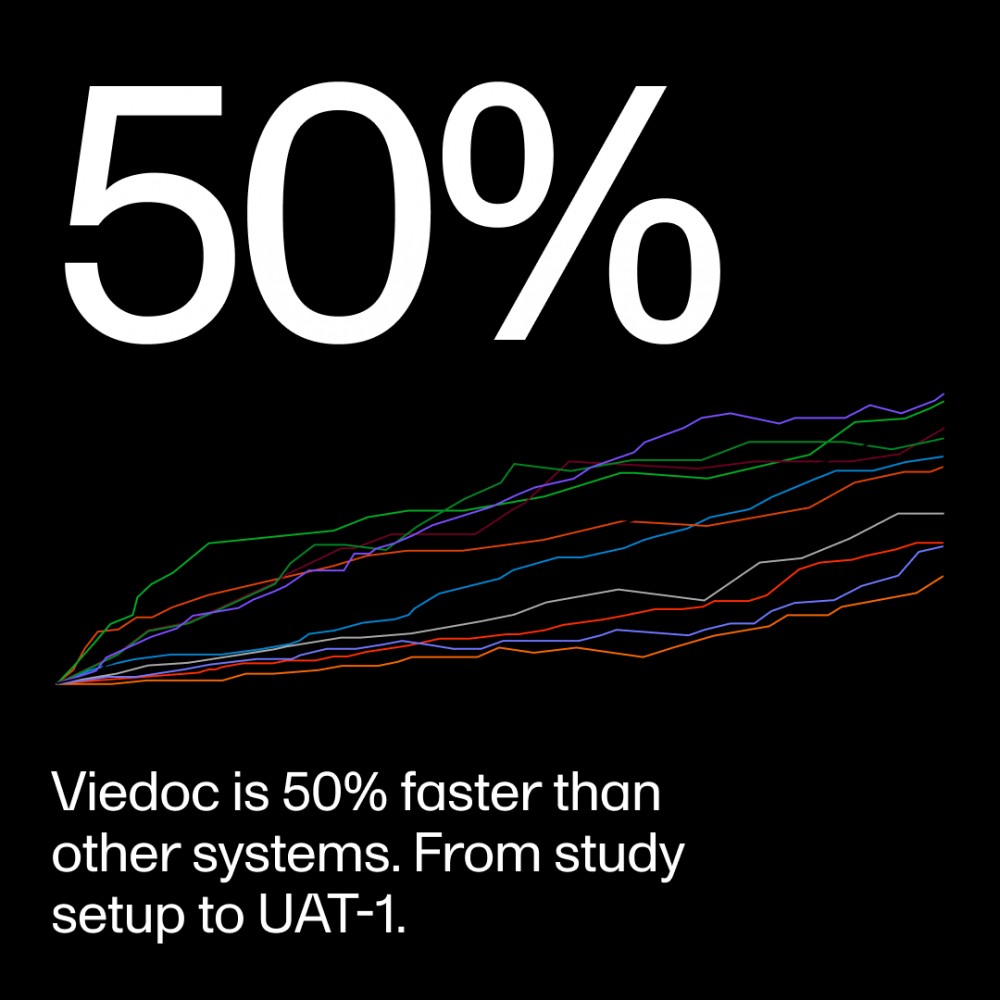
With Viedoc, clinical trial professionals can in-house their trial setup, cutting study timelines and reducing reliance on external vendors. Our platform provides an intuitive interface that supports traditional pathways required for sophisticated study builds while offering up to 50% faster setups. One satisfied user commented, "I'm amazed at how fast I can build my studies using Viedoc."
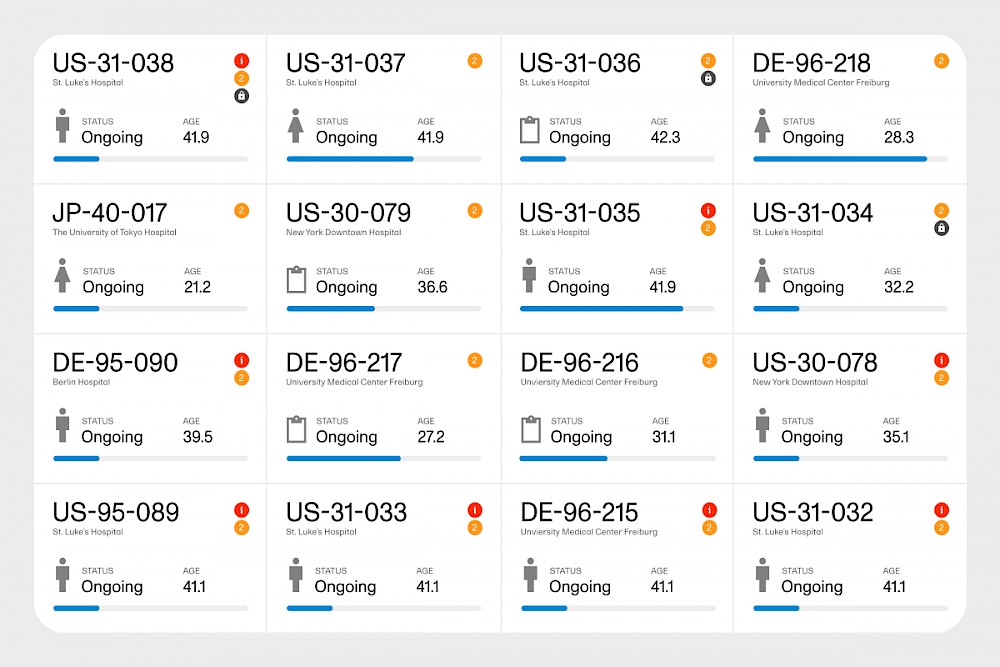
Viedoc is built by clinical research professionals with the needs of the industry in mind. The platform enables clinical teams to work smarter–not harder–by offering features like role-based user guides, configurable user roles, and the ability to delegate activities to different site managers. A happy user shared, "Viedoc has simplified the process of data entry and management, making our clinical trials more efficient and streamlined."

In today's fast-paced world, Viedoc understands the importance of providing a platform that is accessible anytime, anywhere, and on any device. Users appreciate the built-in modern functions, such as auto-reminders, customizable scales, and smooth image capture. One reviewer highlighted this, saying, "The patient-centric features and accessibility on various devices make this platform perfect for our needs."
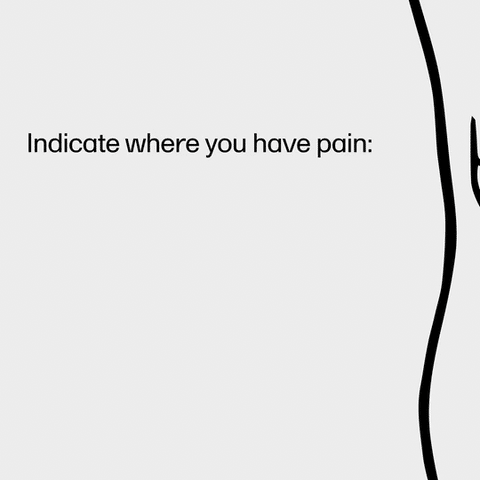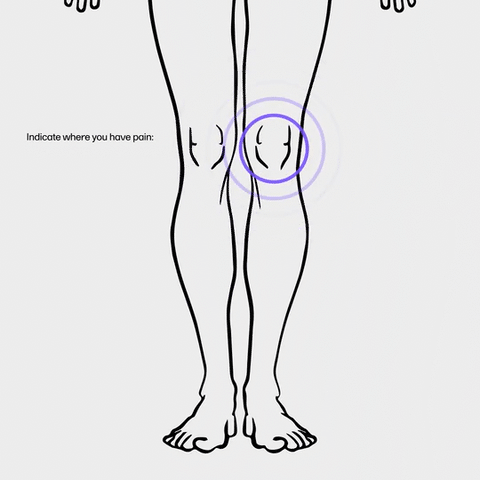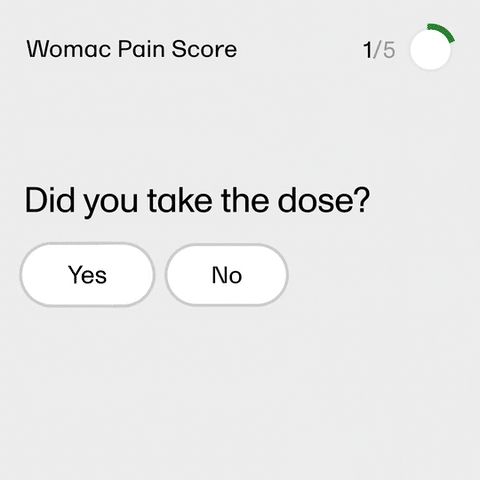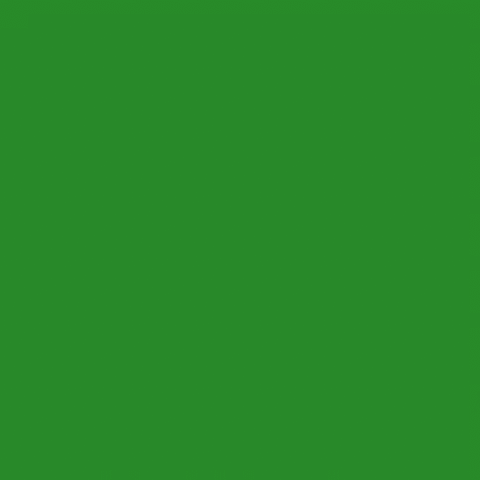
Viedoc supports certified Viedoc Designers by providing ready-made templates and the option to create unique designs or reuse existing ones. The platform is adaptable across studies and scales to each phase, giving users the freedom to manage and configure settings without worry. One reviewer praised this aspect, stating, "The design flexibility and template options have made our study management process so much smoother."
Viedoc holds ISO 27001 certification, offering users peace of mind that their data is secure and compliant with industry regulations. Users can access real-time reports for quicker, deeper insights and enjoy the assurance of continuous data backup and 24/7 protection. As one satisfied customer said, "The data security and compliance features give me confidence in using Viedoc for our clinical trials."

In summary, Viedoc has captured the hearts of clinical trial professionals by addressing key pain points in the industry, streamlining processes, and offering a patient-centric, efficient, and secure platform. The overwhelmingly positive reviews are a testament to Viedoc's commitment to revolutionize clinical trials and empower organizations to bring new treatments to patients faster.
Curious to see Viedoc in action? Our experts would love to give you a tour!

"Viedoc's UI always amazes me. Clean, simple, convenient and user friendly."