
pharmtrace has its own proprietary GCP-compliant image management platform called ERICA, which allows the efficient collection, centralization, and QC of clinical trial images in addition to their reads and reporting. The platform helps to streamline processes as well as provides a complete audit trail to ensure data integrity and transparency for sponsors. While ERICA takes care of the organization and imaging in pharmtrace's trials, Viedoc takes care of the rest.
- Viedoc allows us to offer a comprehensive service package to efficiently organize and manage all aspects of multi-center clinical trials in a manner that fulfills all GCP requirements and meets the needs of our customers, says Antje Hoppenheit, Chief Operating Officer at pharmtrace.


ERICA is a flexible platform for clinical trial image management that combines regulatory compliance for the collection, centralization, and QC of clinical trial medical images with the flexibility of up-to-date image visualization and analysis tools. ERICA provides direct oversight and control of critical clinical imaging-related processes, including the ability to monitor key study performance and quality metrics. ERICA was developed and validated by pharmtrace in real-life clinical trials to correctly document and make clinical imaging GCP-compliant.
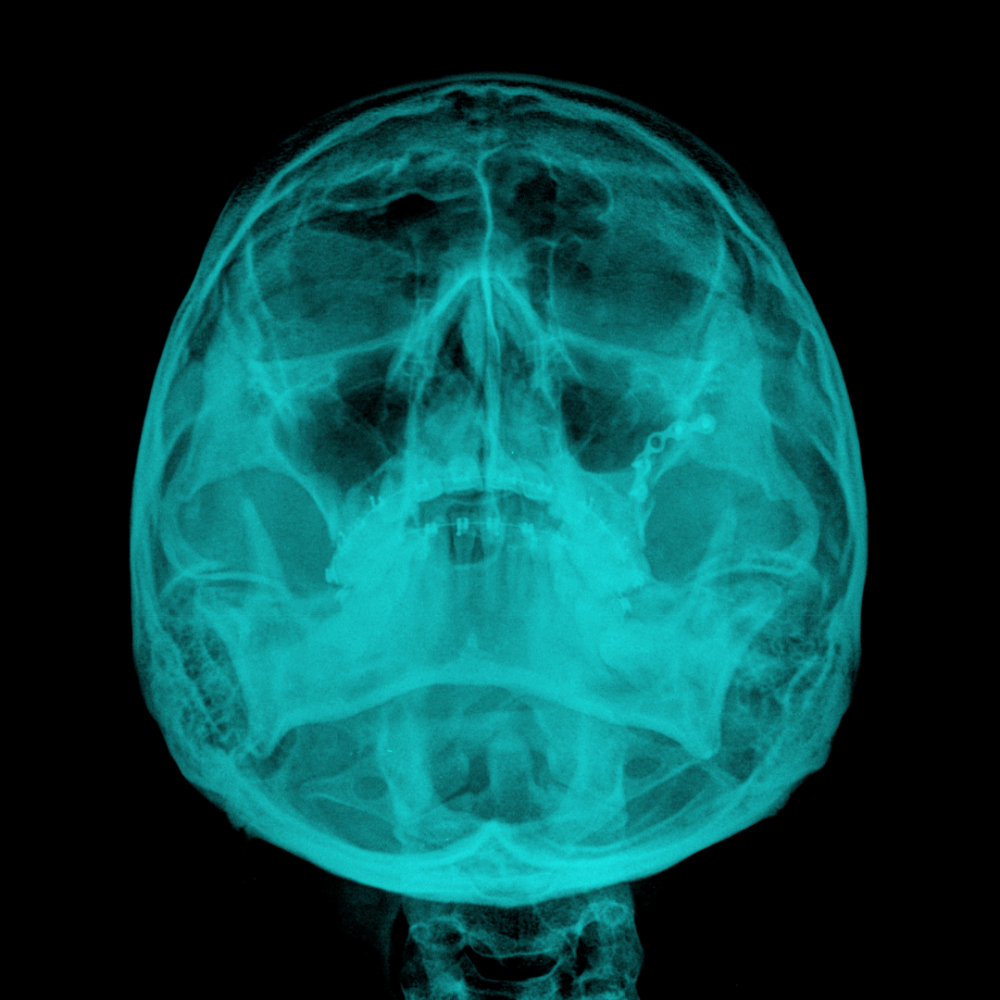
Viedoc perfectly integrates with pharmtrace's ERICA system in the efficient GCP-compliant management of clinical trials with imaging endpoints, radiopharmaceuticals, and radiological interventional procedures. While ERICA is used to make sure that medical imaging in clinical trials delivers robust and meaningful clinical data, Viedoc takes care of the non-imaging aspects of clinical trials so that pharmtrace can meet all the needs of their customers.
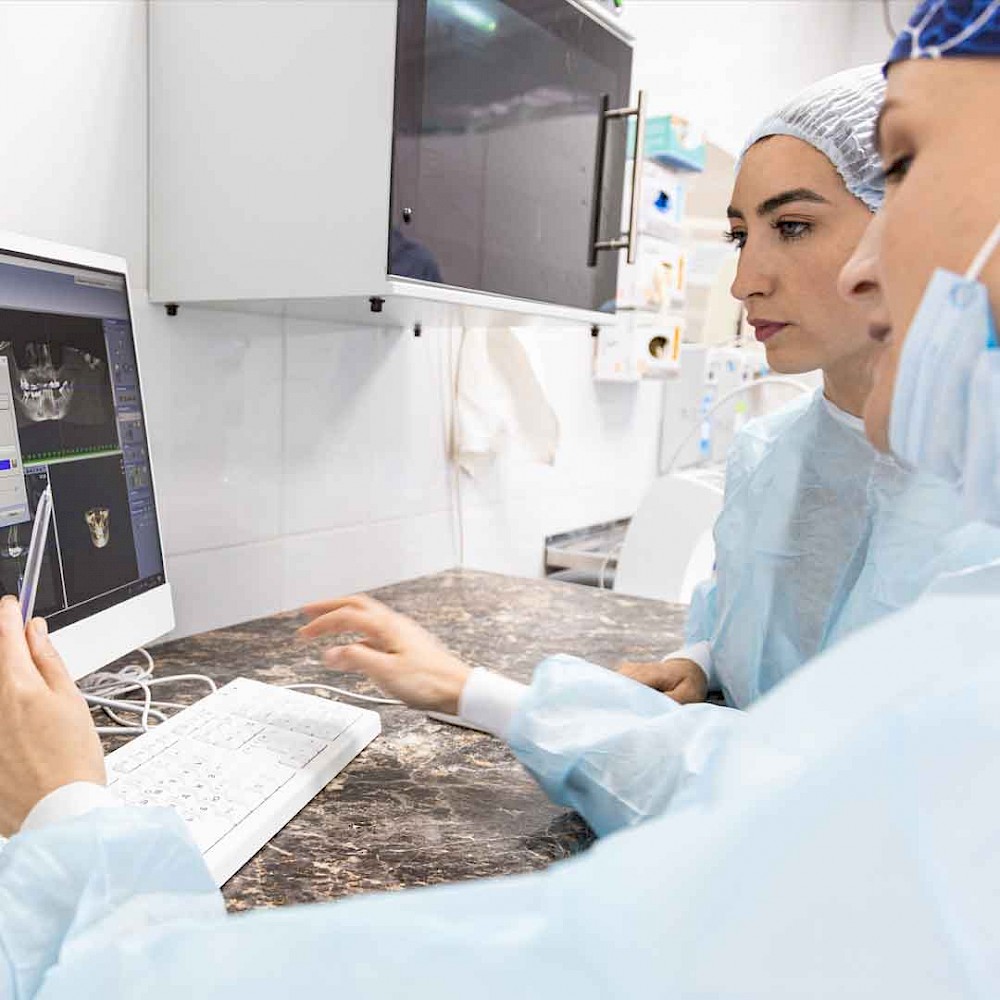
Viedoc offers an easy-to-learn and straightforward interface that is highly appreciated by the site staff, primarily for its intuitive way of use. As a result, less time is needed for internal training. According to Antje, the Viedoc suite provides fewer issues in query management as well as allows central data monitoring. However, if support is needed, Viedoc offers great tech support that is both timely and relevant.
- Viedoc perfectly fulfills our need for risk-based data monitoring in clinical trials, says Antje Hoppenheit, Chief Operating Officer at pharmtrace.
To read more about the Viedoc solution, click here.
To read more about pharmtrace and ERICA, click here.

"Viedoc is an easy-to-use platform made even better with great customer service"