
Keeping up to date with the latest technologies for DCTs and hybrid style trials keeps your studies recruiting and retaining patients. Viedoc offers its own solution for DCTs and hybrid trials through Viedoc Me and Viedoc Connect.
The 5 key factors below give you an understanding of how DCTs can alter and lead to better clinical trials for your studies.
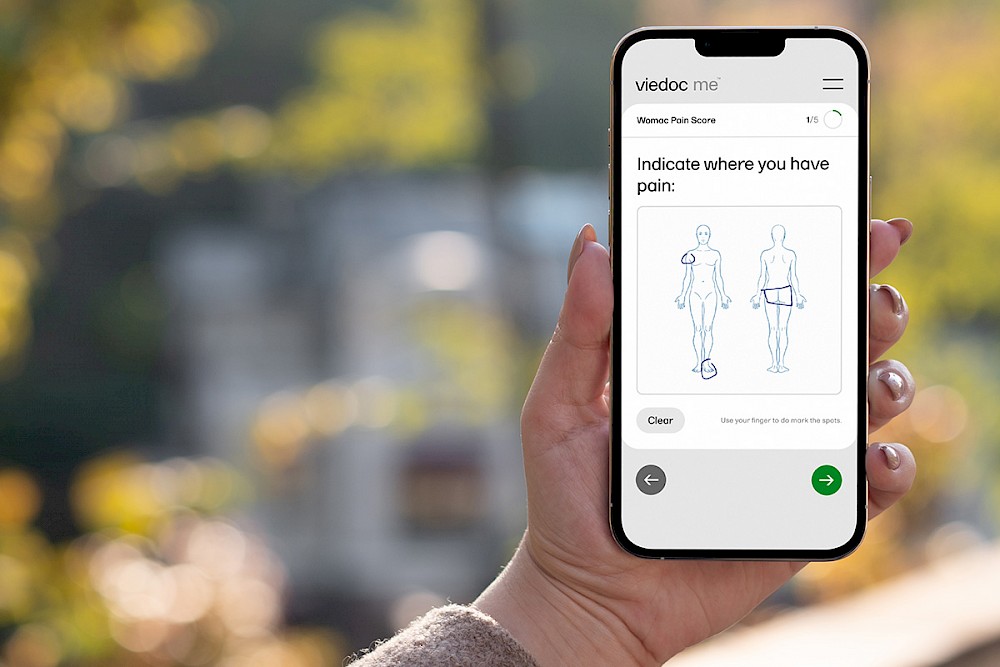
DCTs and hybrid trials put the patient first. Using an app and a smart device, a patient can fill out forms, give consent, schedule visits with medical staff, and in the case of Viedoc’s own ePRO (electronic patient reported outcome) solution, Viedoc Me, they can speak directly with a physician over the app through Viedoc Connect.
Through the patient centered approach DCTs provide, you also reduce costs, and hardships on site staff. Where standard COAs (clinical outcome assessment) are very manual and labor intensive, an eCOA (electronic-clinical outcome assessment), like Viedoc Me, can directly capture the trial data on a patient's smart device, for example.
Different people have different responses. With greater technological and mobile access, trials can now recruit from a wider ranging pool of candidates. With more candidates from more diverse backgrounds, not only do we increase health equity, but we also increase health quality. Viedoc’s DCT/hybrid solution, Viedoc Connect, puts the power of diversity in the pockets of patients who use Viedoc Me and gives the power of better data and management to clinical trials.

Recruiting clinical trial patients is difficult. You want to get the right patient for the right study. With DCTs, screening patients is much faster, flexible, and best of all—cheap! Patients also save time by using their phones or tablets to get through forms and documents, even undergo the eConsent process—a feature that is part of Viedoc’s ePRO/eCOA solution, Viedoc Connect.
Similarly, a patient that has a specific and rare condition, but lives extremely far from trial sites, is now accessible. DCTs and ePRO solutions, together, allow for greater possibilities of recruitment as the patient experiences less risk, can connect more quickly with medical staff, and has more opportunities to communicate stronger data points.
The smoother a trial runs the better for the experience of the patient, the more likely they will stick with the trial. When they can have an engagement with the trial that is focused more on them, like being able to use their own devices to track data, or managing a study device, that gives them a greater sense of ownership. Viedoc offers support for eCOA through Viedoc Me and Viedoc Connect, making it easier for the patient to get through the trial, in turn boosting your trial’s retention. During the Covid-19 pandemic it was found that trial retention rates were nearly 90%! That’s the power of DCTs.

When it comes to better recruitment, better retention, and consistency from patients, your trial will get the quality data it needs to make an impact. Fitness devices, smart watches, health tracking apps, and the IOT (internet of things), allow for greater access to passive data streams.
Also, through eDiaries/journals, and technologies like Viedoc Connect, patients can report random events, contact clinical staff and set up a video call in a matter of seconds. Devices offer the luxury of needing little technical acumen on behalf of the patients, as well.
With a Decentralized and hybrid solution like Viedoc Me and Viedoc connect, patients are taken through an intuitive UI which guides them through every step of the trial process. It takes out the technical aspects and reduces the complexity. This means less mistakes, faster patient access, and greater access to better data streams.
Come learn more about how Viedoc’s DCT/Hybrid solution can transform your trial.


"What I like best about Viedoc is their approachable support and "can do" attitude. Their service has always been personal and reliable."