Choosing the right electronic data capture (EDC) system is one of the most important decisions in planning a clinical trial. The right platform can streamline study setup, improve data quality, and reduce delays. The wrong choice can lead to inefficiencies, spiralling costs, and regulatory headaches.
With dozens of vendors offering a wide range of features – from intuitive interfaces and rapid study builds to advanced integrations and global scalability – it can be difficult to know where to begin.
Here’s a closer look at some of the leading providers and what they offer.
1. Viedoc
2. Medidata
3. Veeva
4. Oracle Health Sciences
5. Castor
6.Medrio
7. ClinCapture
8. OpenClinica
9. REDCap Cloud
10. Zelta
Key evaluation criteria for EDC systems
1. User experience
The intuitiveness of the user interface and the ease of onboarding for sites and patients.
2. Regulatory compliance
Compliance with 21 CFR Part 11, GDPR, HIPAA, regional regulations, and audit trail capabilities.
3. Speed to deploy
Study build time, database go-live speed, and flexibility for mid-study amendments.
4. Pricing
Transparency of pricing models, potential hidden fees, and total cost of ownership.
5. Integration capabilities
API availability, documentation, and integration with EHR/EMR, CTMS, RTSM, and ePRO.
6. Scalability and performance
Support for complex trials, global deployment, and system uptime.
1. Viedoc
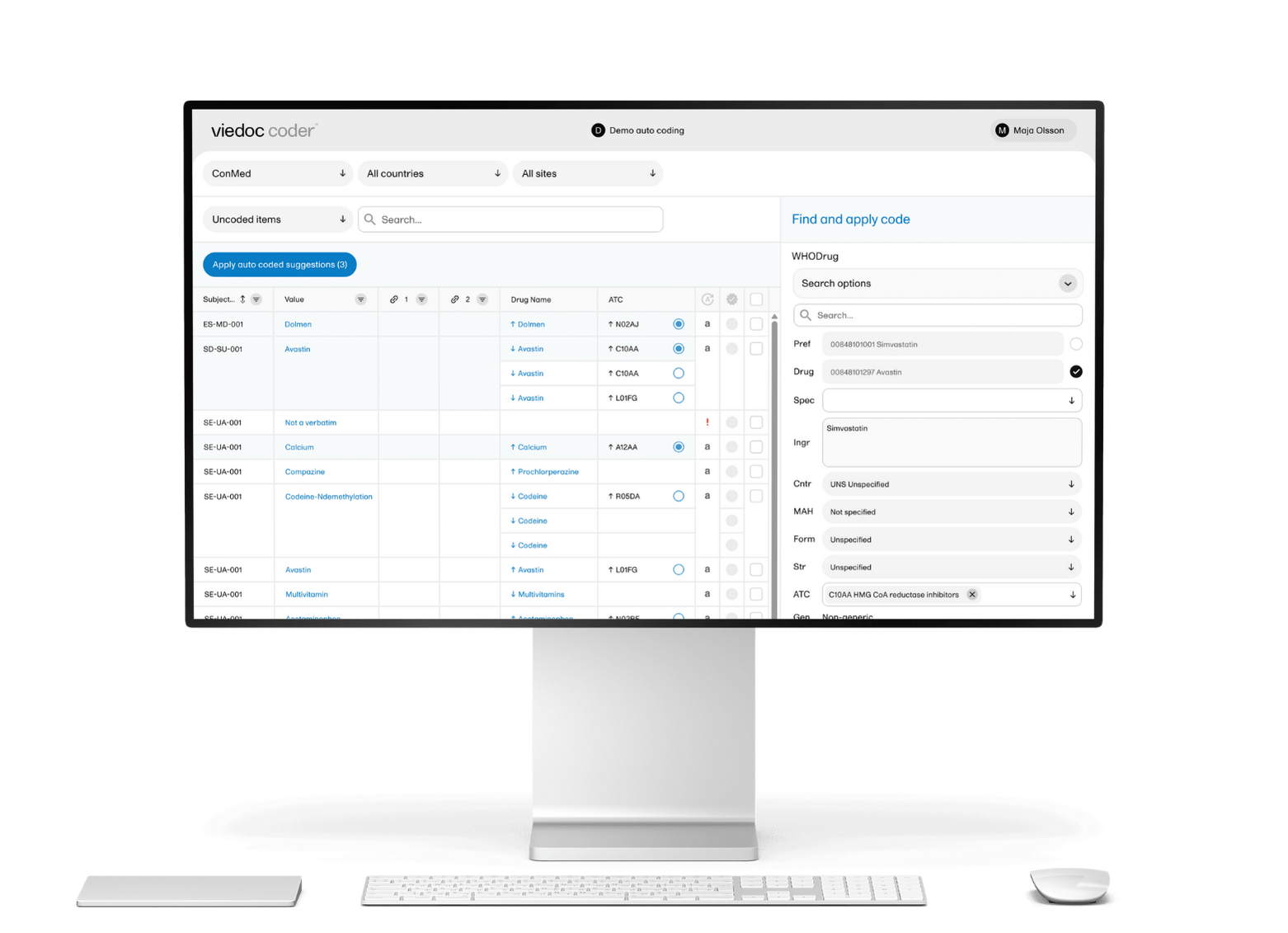
Viedoc's EDC clinical trial software combines enterprise-grade capabilities with exceptional flexibility, such as modular pricing. Trusted in over 7,000 trials across 75+ countries — and named a Leader by Everest Group in its 2024 Life Sciences EDC Products PEAK Matrix® assessment — Viedoc supports all trial phases and therapeutic areas.
Key strengths:
- Faster study builds with drag-and-drop interface
- 99% uptime ensures continuous availability
- No hidden costs
- Built by CRO professionals for seamless workflow integration
- Make mid-study changes without system interruptions
- Meets FDA 21 CFR Part 11, GDPR, HIPAA, and regional requirements in Japan and China
- Launch studies in as little as 8 weeks
Notable features:
- Unified eClinical suite including EDC, ePRO/eCOA (Viedoc Me), RTSM (Viedoc Logistics), and eTMF
- Support for 40+ languages with localization in 10+ interface languages
- 24/7 technical support across global offices
- Pre-configured templates in CDASH format
- Native SDTM transformation capabilities
Viedoc customer results:
Qmed: Achieved faster data collection, reduced data entry errors, real-time monitoring capabilities, and increased data accessibility when transitioning from paper-based processes
Chordate Medical: Achieved high patient compliance rates and eliminated manual data entry workload for clinics
Tecro Research & LMU Klinikum: Achieved faster data imports and improved quality control for their multi-country TB trial across 8 sites in 6 African countries
2. Medidata
Rave is Medidata’s comprehensive EDC platform. It captures, manages, and reports clinical trial data across all phases and therapeutic areas, facilitating efficient data handling and real-time decision making.
Key strengths:
- Extensive industry experience with over 36,000 trials conducted
- Comprehensive platform with integrated safety and coding modules
- AI-powered study design capabilities
Notable features:
- Large user base
- Advanced analytics and reporting tools
- Strong integration with other Medidata solutions
3. Veeva
Part of Veeva's comprehensive Clinical Platform, Veeva Vault EDC is a modern, cloud-based EDC solution. It offers an end-to-end environment for collecting, reviewing, and processing site-reported patient trial data, catering to complex studies such as multi-arm and adaptive trials.
Key strengths:
- Zero downtime for amendments
- Strong integration within the Veeva ecosystem
- Modern cloud architecture
Notable features:
- Drag-and-drop study designer
- Dynamic visits and forms
- Seamless CTMS integration
4. Oracle Health Sciences
Oracle InForm is a comprehensive EDC platform supporting global clinical trials. It has extensive integration capabilities and mobile device support, making it suitable for large-scale studies.
Key strengths:
- Extensive global footprint
- Competitive pricing
- Strong integration capabilities
Notable features:
- Mobile device support
- Comprehensive trial management modules
- Audit trail and regulatory compliance tools
5. Castor
This modern, API-first platform is recognized for its rapid study deployment and strong eCOA and eConsent capabilities. Castor EDC caters to both academic and commercial research needs.
Key strengths:
- User-friendly, no-code design
- Comprehensive data integration from wearables and other devices
- Real-time visibility through a study health dashboard
Notable features:
- No-code platform design
- eConsent/eCOA support
- API-first platform with integration options
- Compliant with 21 CFR part 11, ICH e6 GCP, GDPR and HIPAA
6. Medrio
Medrio is a unified platform for EDC, eCOA/ePRO, eConsent, and RTSM, prioritizing simplicity without compromising functionality. Its EDC supports both online and offline data capture, offering intuitive user interfaces for sites and participants.
Key strengths:
- User-friendly interface
- Flexible and scalable
Notable features:
- Comprehensive eClinical suite
- Global language and localisation support in 100+ countries
- Native SDTM transformation
7. ClinCapture
ClinCapture provides a Virtual Data Capture suite for decentralised trials with the Captivate EDC platform. It offers an open-source foundation with offline capabilities, catering to various study complexities.
Key strengths:
- Focus on decentralized trials
- Open-source foundation
- Cross-study and cross-site reporting capabilities
- APIs and web service integration are available for external systems like CTMS, lab systems, and safety systems
Notable features:
- Captivate® EDC platform
- VDC ePRO/eCOA and RTSM modules
- Private cloud deployment options
8. OpenClinica
As the name suggests, OpenClinica is an open-source EDC system that accelerates clinical trial data management with automation, compliance, and scalability. Designed to make trials more efficient and accessible, the platform combines an intuitive, no-code interface with powerful capabilities for study design, data capture, compliance, and reporting.
Key strengths:
- Open-source flexibility with enterprise option
- Strong regulatory compliance
- Scalable for multi-site studies
Notable features:
- EDC and eCRF management
- Audit trail and reporting tools
- Integration with third-party systems
9. REDCap Cloud
REDCap Cloud is a secure, cloud-based platform ideal for academic and non-profit research. The EDC system enables researchers to collect, integrate, and analyze data across studies seamlessly — providing real-time insights and facilitating smarter decision-making throughout the trial lifecycle.
Key strengths:
- Supports mid-study changes to CRFs or visits
- Rapid setup and study deployment
- Strong data management capabilities
Notable features:
- eConsent and survey management
- API access for integrations
- Audit trail and compliance tools
10. Clario
Clario is a global provider of clinical trial technology, offering an EDC platform alongside solutions for imaging, eCOA, cardiac safety, and endpoint data management.
Key strengths:
-
Established global provider with extensive industry experience
-
Strong regulatory compliance
-
Integrated solutions for imaging, eCOA, and endpoint data capture
- 24/7 customer and patient support
Notable features:
-
Customizable site workflows and dashboards
-
Audit trail and compliance monitoring tools
-
Seamless integration with IRT, analytics, and reporting modules
The top 10 EDC systems at a glance
| Vendor | Usability | Compliance | Speed to deploy | Pricing | Integration | Scalability |
| Viedoc | Drag-and-drop interface, intuitive, and user-friendly |
21 CFR Part 11, GDPR, HIPAA |
Fast builds | Transparent, no hidden costs | API, CDASH, SDTM, eTMF, RTSM, ePRO | 99.99% uptime, supports all trial phases, global reach |
| Medidata Rave | Mature platform | 21 CFR Part 11, GDPR, HIPAA; | Industry standard, but setup can be complex for large studies | Enterprise pricing | Deep integrations within Medidata Clinical Cloud | Supports large, global, multi-phase studies |
| Veeva Vault EDC |
Modern UI, drag-and-drop interface, |
21 CFR Part 11, GDPR; full audit trail | Fast builds | Subscription-based | Seamless integration within Veeva Vault (CTMS, eTMF, payments) | Cloud-native, flexible for global studies |
| Oracle | Established platform with mobile support | 21 CFR Part 11, HIPAA, GDPR | Supports global deployments | Tailored pricing | Strong EHR/EMR, CTMS, RTSM integrations | Enterprise-level scalability and uptime |
| Castor | No-code, user-friendly | 21 CFR Part 11, GDPR, HIPAA | Built for quick deployment | Transparent pricing available online | API-first; integrates with EHR/EMR, wearables, analytics | Supports both academic and commercial trials globally |
| Medrio | Simple, intuitive; mid-study edits | 21 CFR Part 11, GDPR; audit trails | Fast deployments | Transparent pricing model | API, RTSM, eConsent, ePRO, eCOA | Scales across Phase I–IV, multi-language support |
| ClinCapture | Focused on decentralized trials | 21 CFR Part 11, GDPR | Flexible deployment; private cloud options | Open-source base | VDC suite with RTSM, ePRO/eCOA modules | Scales from small to complex decentralized trials |
| OpenClinica | Open-source flexibility | 21 CFR Part 11, GDPR; strong audit trail | Moderate build speed | Enterprise pricing | EHR/EMR, CTMS, analytics integrations | Proven scalability across multi-site studies |
| REDCap Cloud | Survey-style, intuitive for non-profits | 21 CFR Part 11, HIPAA, GDPR | Rapid deployment | Enterprise pricing | APIs for integrations; survey/eConsent | Cloud-based, well-suited for academic/global research |
| Zelta | Customizable workflows; site-focused usability | 21 CFR Part 11, ICH-GCP; strong audit trail | Flexible deployments | Enterprise pricing | Integrates with IRT, eCOA, imaging, and analytics | Global reach |
Making the right choice
The right EDC system balances usability, compliance, speed, cost, integration, and scalability. By aligning platform capabilities with the specific needs of your study, you can streamline operations, safeguard data quality, and avoid costly delays.
/female-doctor-with-tablet.jpg?width=624&height=405&name=female-doctor-with-tablet.jpg)

/new-year.jpg)