Ozilia® Migraine: A drug-free neuromodulation solution
Chordate Medical introduced its patented treatment method, Ozilia® Migraine, in 2021. It uses a CE-marked device that can replace existing medications with few to no side effects. Using neuromodulation, the Chordate solution retrains the autonomic reflex that leads to migraines, offering a non-pharmacological treatment approach.
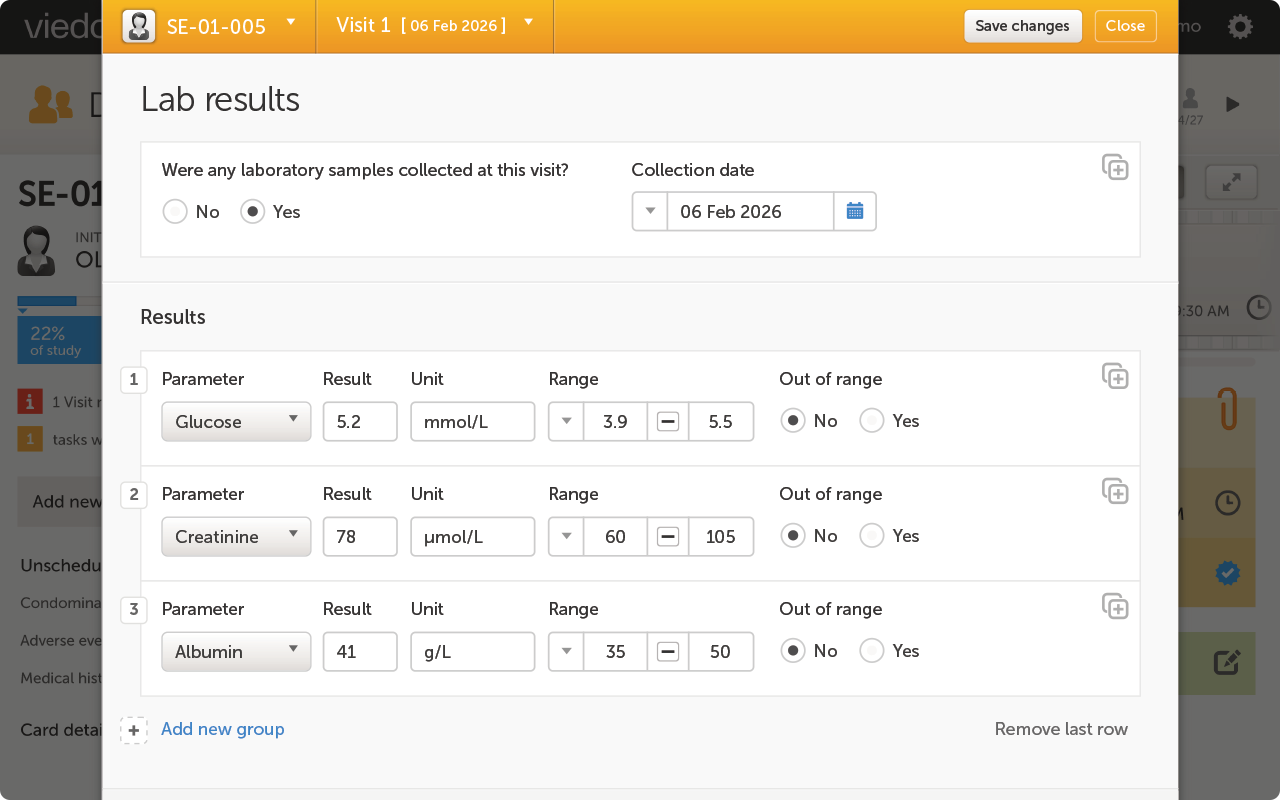
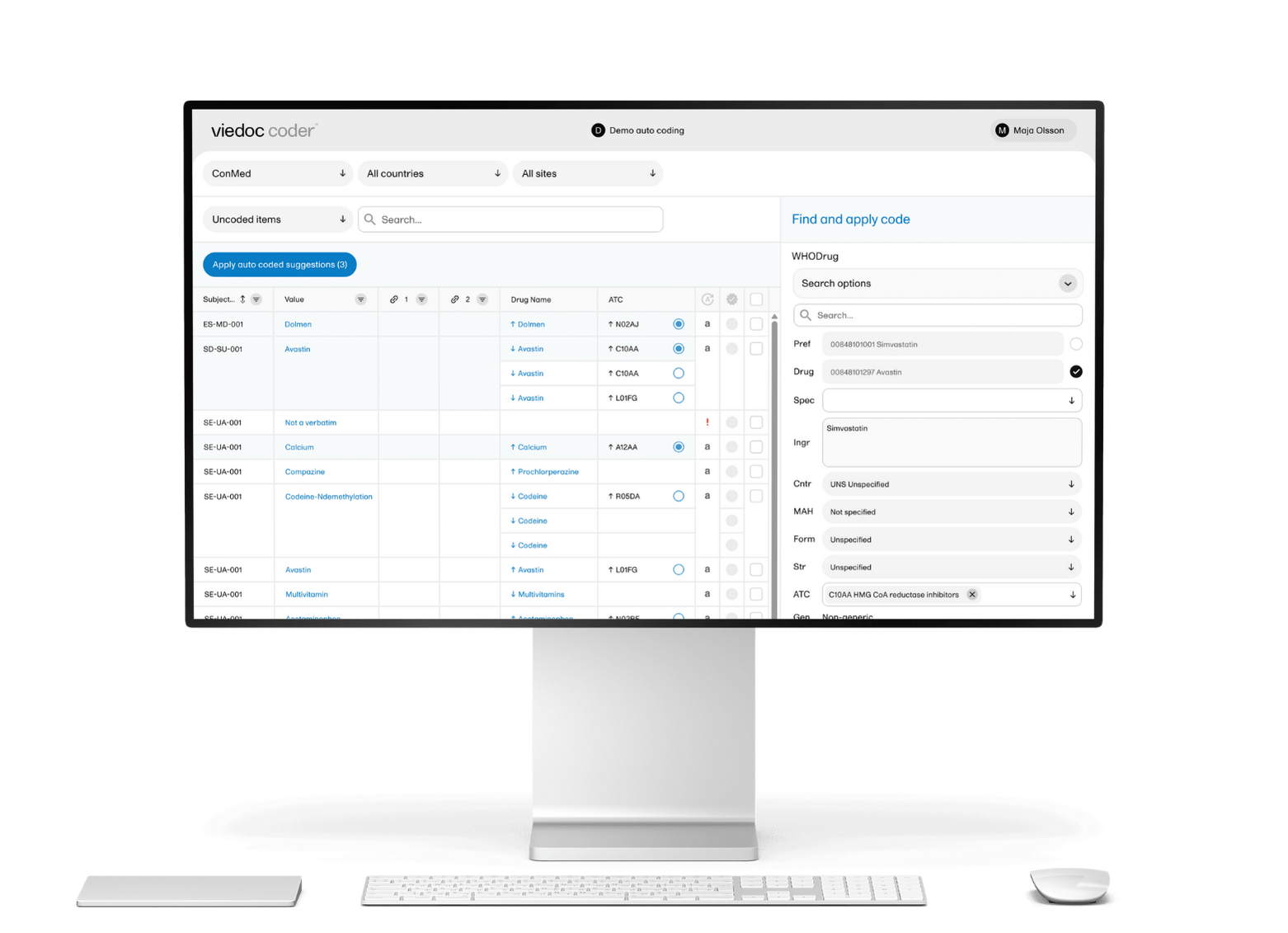
To ensure both the safety and effectiveness of Ozilia® Migraine, Chordate established a clinical trial across 10 centers in England, Germany, and Italy, using Viedoc’s electronic patient-reported outcomes (ePRO) solution, Viedoc Me, to capture and manage patient data. The long study duration and international nature of the trial made patient recruitment and engagement challenging. By providing a simple, straightforward user experience, Viedoc Me supports more reliable results, giving Chordate’s study teams better data to analyze.
The treatment’s aim is to reduce the symptoms and frequency of migraine attacks without using medication. Some patients are hesitant to take migraine medication due to the side effects and ongoing cost, so Chordate’s CE-marked treatment provides a genuine alternative.
Chordate’s method of mechanical neurostimulation takes just 20 minutes in a clinical setting and has been shown to provide long-term migraine relief, with occasional follow-up treatments. A randomized, sham-controlled, double-blind study was needed to validate the initial findings, which were an outgrowth of using the same device on patients with chronic rhinitis. Ozilia® Migraine has been clinically proven effective for migraine treatment in peer-reviewed research published in the journal Neurology, highlighting its role as a safe, non-drug neuromodulation therapy.
/doctor-smiling-patient.png?width=1280&height=800&name=doctor-smiling-patient.png)
/female-doctor-glasses-laptop.png?width=1000&height=667&name=female-doctor-glasses-laptop.png)
/doctor-patient-examination-table.png?width=1000&height=667&name=doctor-patient-examination-table.png)

/female-patient-doctor-stethoscope.png?width=1000&height=667&name=female-patient-doctor-stethoscope.png)
/female-doctor-with-tablet.jpg)