Accurate, compliant data capture drives successful clinical trials. The electronic Case Report Form, or eCRF, supports this process for high-quality data, trial after trial.
What is an eCRF?
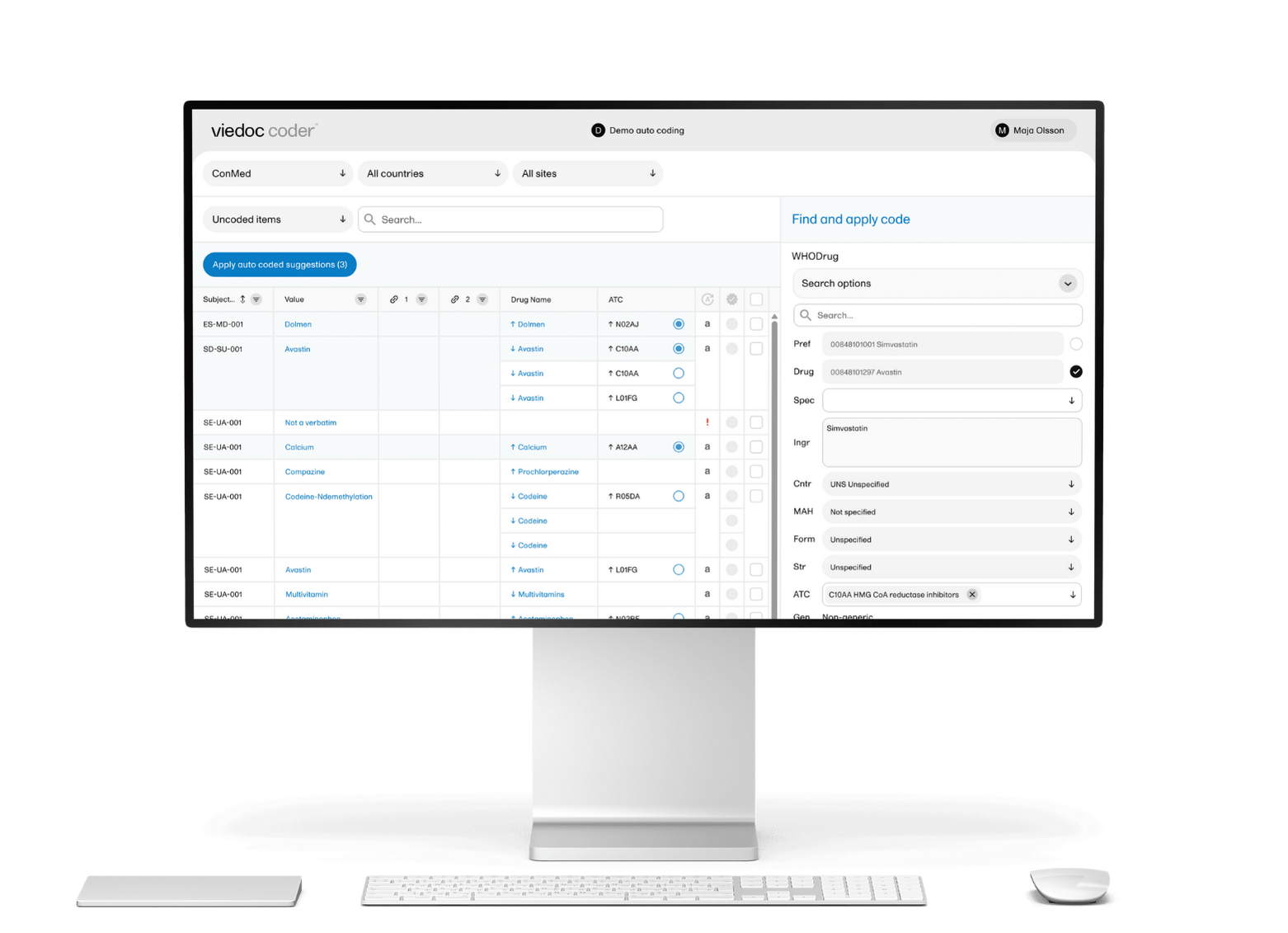
An eCRF is a digital form for clinical trials that replaces paper-based documentation at research sites. Study teams configure the eCRF based on set guidelines to collect patient data in a structured, consistent format. Entering data in this way enables real-time validation, automated logic checks, and immediate flagging of protocol deviations.
Using eCRF in clinical trials reduce transcription errors, minimize missing data, and cut down the time spent on query resolution. They also enforce standards that align with regulatory frameworks, like FDA 21 CFR Part 11 and ICH GCP.
How to approach eCRF design
Clean data begins with the eCRF. Well-designed eCRFs reduce risk, accelerate timelines, and improve data quality across every study.
Effective eCRF design focuses on:
- Form layout. Users should be able to navigate your forms with minimal training.
- Data integrity. Built-in validation logic ensures accuracy.
- Compliance support. Forms track every change, capture electronic signatures, and meet protocol requirements.
Every decision during design – like field types, conditional logic, and visit scheduling – affects downstream performance.
What to expect from eCRF systems
Modern eCRF software provides the infrastructure for high-quality data capture and ensures regulatory compliance from the first patient to the final lock.
Your system should offer:
- Custom form builders that reflect protocol complexity
- Real-time validation logic that prevents errors at the point of entry
- Audit trails that log changes, timestamps, and user actions
- Role-based access control to protect data integrity
- Electronic signature functionality that supports compliance
These features let you define how sites enter and manage data while maintaining full traceability – something regulatory agencies expect.
In addition to data capture and traceability, you also need tools that support compliance oversight and site readiness. For example, Viedoc Clinic provides:
- Expanded role and permission management to align access with site responsibilities and training status
- Training documentation tools that help prove site readiness
- Real-time dashboards for monitoring data entry and protocol adherence
Some eCRF software, like Viedoc Clinic, fully integrates with EDC clinical trial software, which reduces manual or duplicate work and increases efficiency.
eCRF vs. EDC
eCRF vs. EDC, CTMS vs. EDC, eTMF vs. EDC – the list of comparisons goes on. Many people use eCRF and EDC interchangeably, but they refer to different things.
| eCRF | EDC |
| Digital form to enter data | Software platform for the entire data capture process |
| Focused on structured data collection | Manages forms, validation, audit trails, user access, exports, queries |
| Resides inside the EDC system | The system that houses the eCRFs |
The eCRF is the form that captures clinical trial data. The EDC manages the data – storing, processing, and organizing it across the study. Both are essential, but serve distinct purposes.
In platforms like Viedoc, the eCRF comes fully embedded in the EDC management system. That means fewer integration issues, faster setup, and a smoother experience for your sites and data managers.
eCRF example
You could use site-specific eCRFs to address regional regulatory requirements and language differences in a multi-country trial.
Dynamic logic lets you adapt forms by country, so each site sees a localized, native-language version of the eCRF. Despite the differences, the system will capture all data in a unified structure, allowing for global oversight and analysis.
This configuration, alongside built-in data validation, enables real-time interim analysis.
Why eCRFs are critical for clinical trial success
Every successful trial starts with clean, consistent data. An eCRF ensures you collect that data correctly, improves protocol compliance, speeds up data review, and gives you complete visibility into trial performance.
By embedding compliance features and supporting user accountability, modern eCRF systems help you stay inspection-ready without extra work. And, when fully integrated into your EDC or broader eClinical stack, you can manage your entire study from one platform.


/new-year.jpg)
/female-doctor-with-tablet.jpg)