
June 30, 2025

June 30, 2025
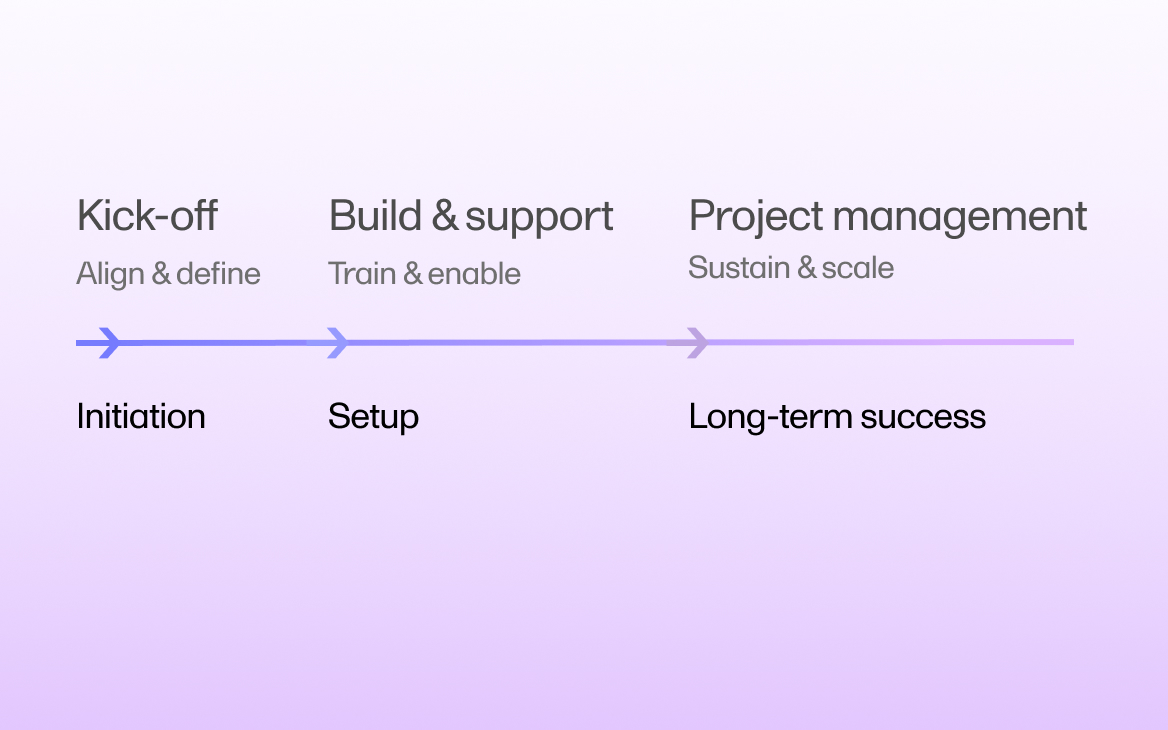
June 25, 2025

June 19, 2025

June 18, 2025

June 16, 2025
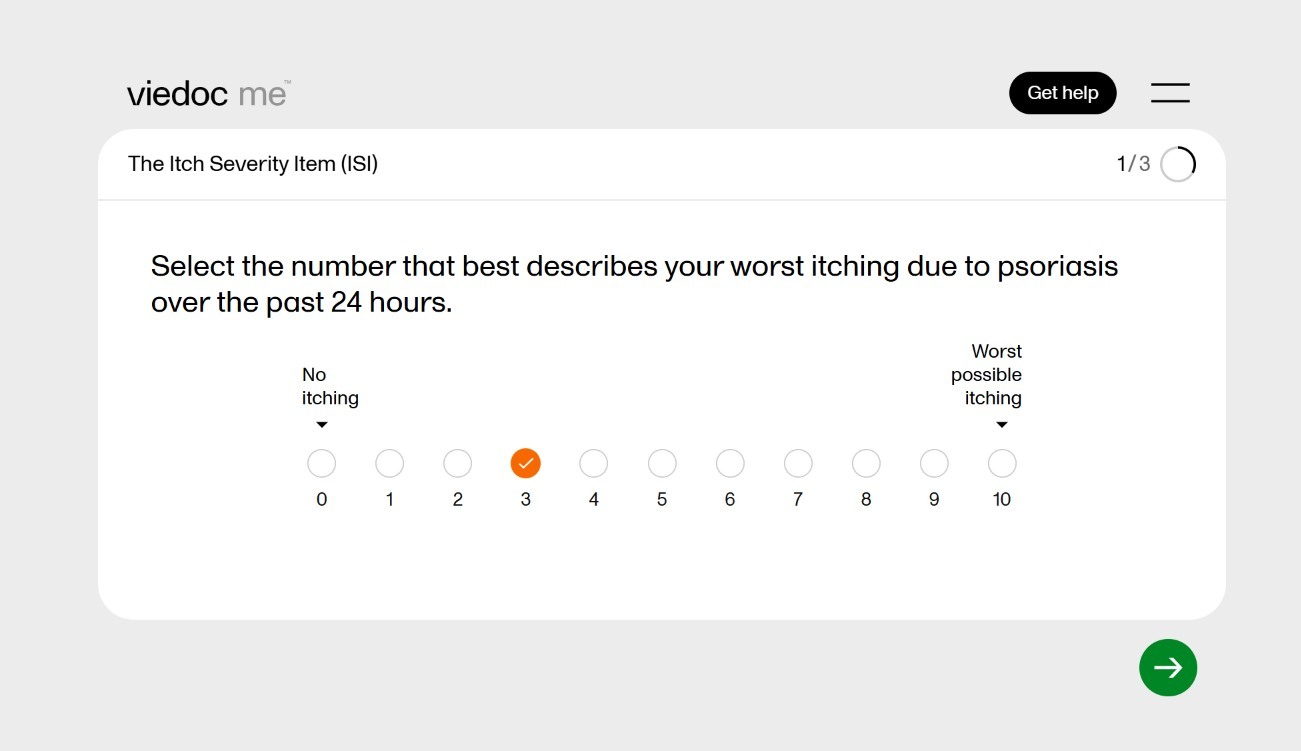
June 12, 2025

May 22, 2025

May 15, 2025

May 08, 2025

May 05, 2025

April 29, 2025