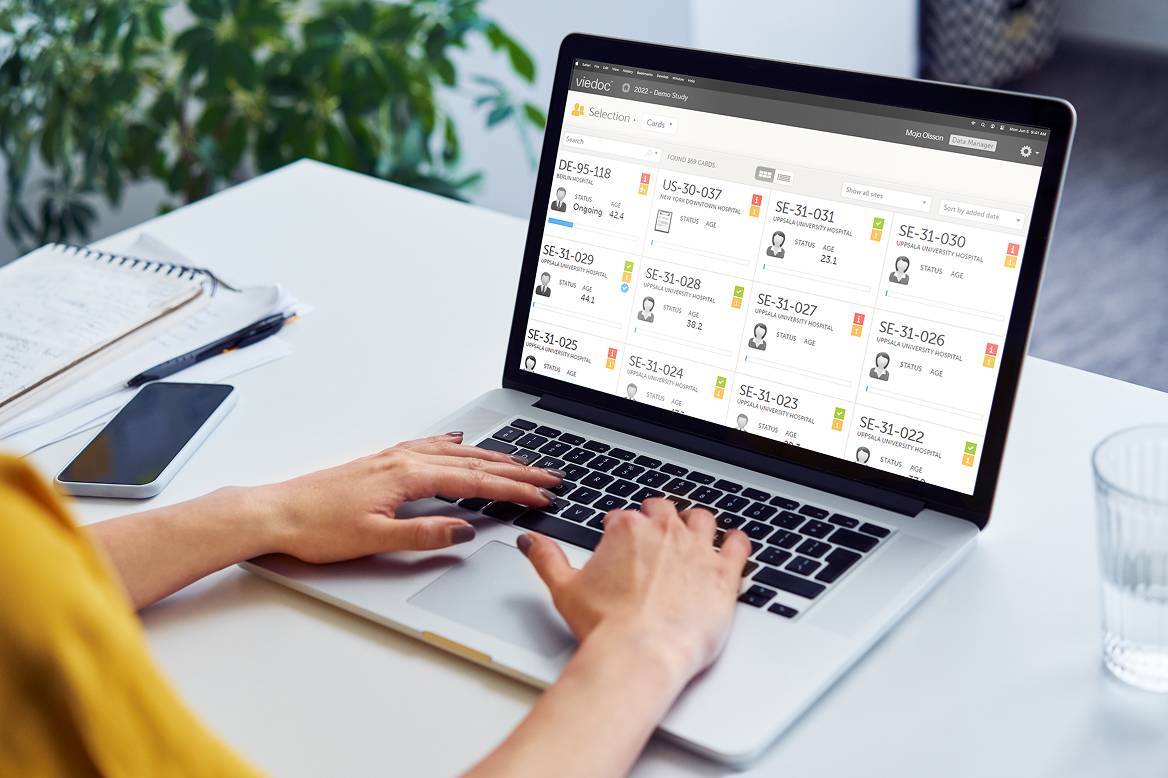
Discover how Viedoc, BSI Life Sciences, and LINK Medical work together to deliver seamless integration—resulting in smoother workflows, stronger site collaboration, and more efficient clinical trials. In this joint testimonial, our partners share real examples of how the three-way collaboration adds value to studies, enhances user experience, and supports the evolving needs of modern clinical research.
December 03, 2025

/female-doctor-with-tablet.jpg)