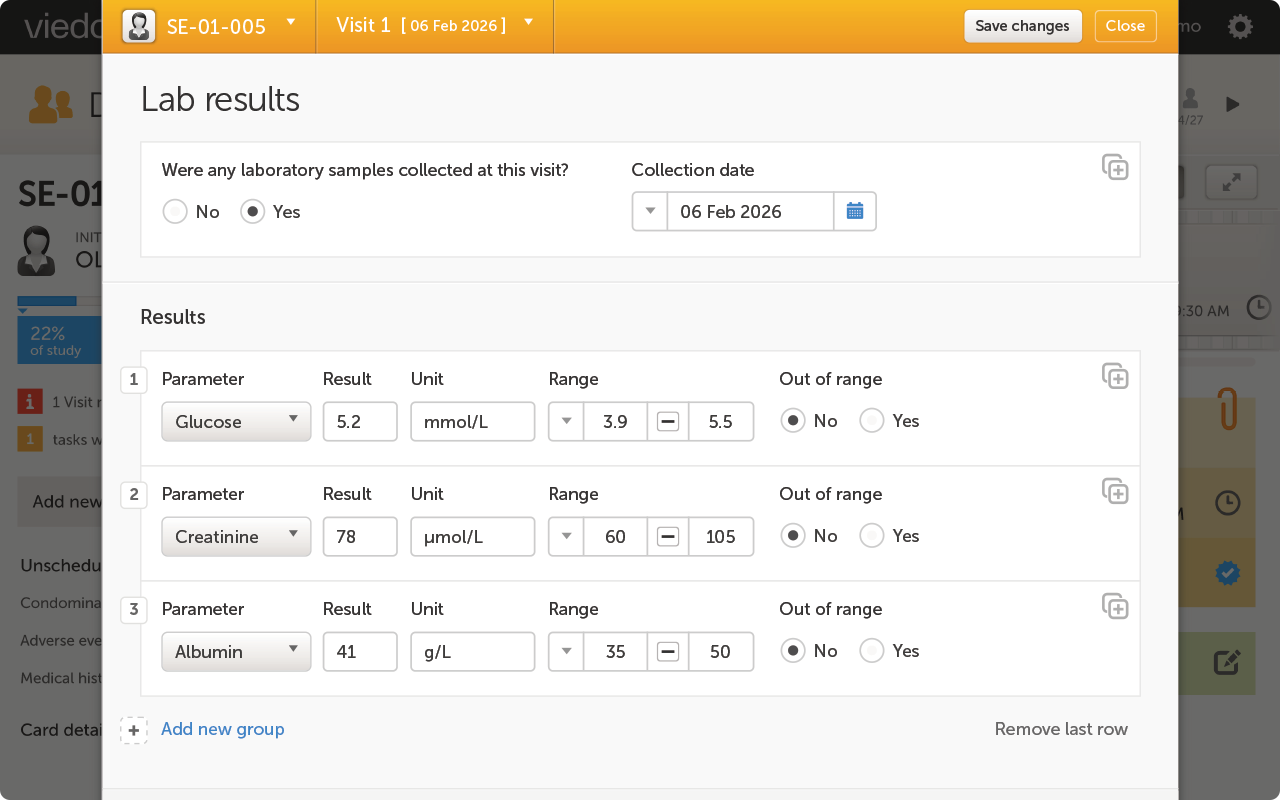
Get expert clarity on the latest ICH E6(R3) draft guidance and its impact on clinical trials.
In the kickoff session of our new LinkedIn Live series, Viedoc experts break down what the E6(R3) updates really mean — and how you can prepare. Whether you're a sponsor, CRO, or tech provider, this conversation is packed with practical insights to help you align with the new guidance.
February 25, 2026




/female-doctor-with-tablet.jpg)