
Through one modern, streamlined interface, Viedoc Clinic allows you to efficiently access, manage, review, and share clinical trial data—from any device, at any time.
A subject display with clearly labeled cards allows you to instantly locate and select specific subjects.
Working on a tablet device? You’ll enjoy Viedoc Clinic’s interface, designed for maximum ease of use whichever platform you’re on.

From missing data to signing, review, and lock status, the events overview makes it easy to identify and address patients and events that require attention.
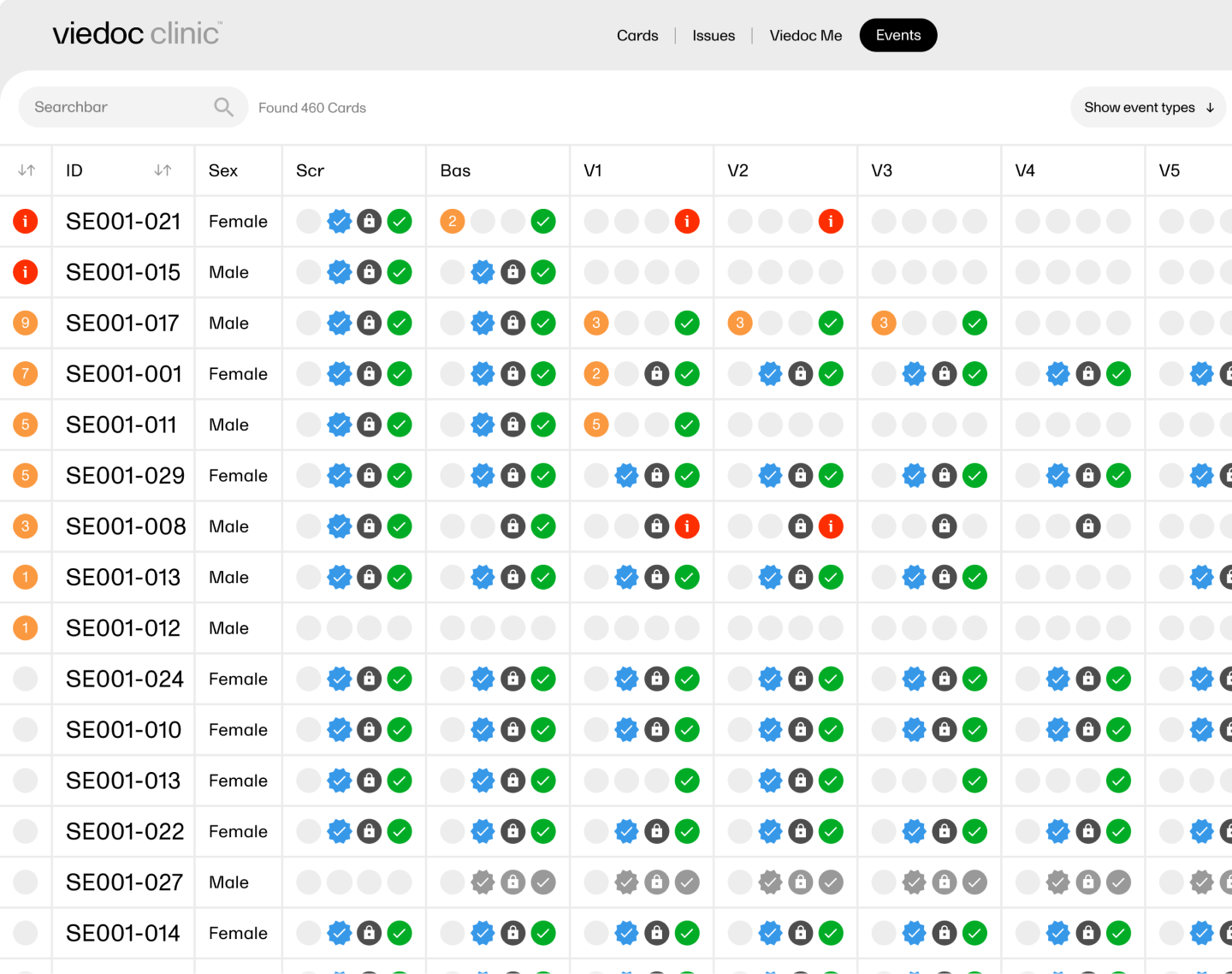
According to your user role, you’ll be provided with prompts helping you take your next step: sign data, resolve a query, complete missing data, etc.
Knowing that reliable data quality is essential to any study, we’ve designed the metrics page to provide you with fresh, real-time data – on study, country, or site level.
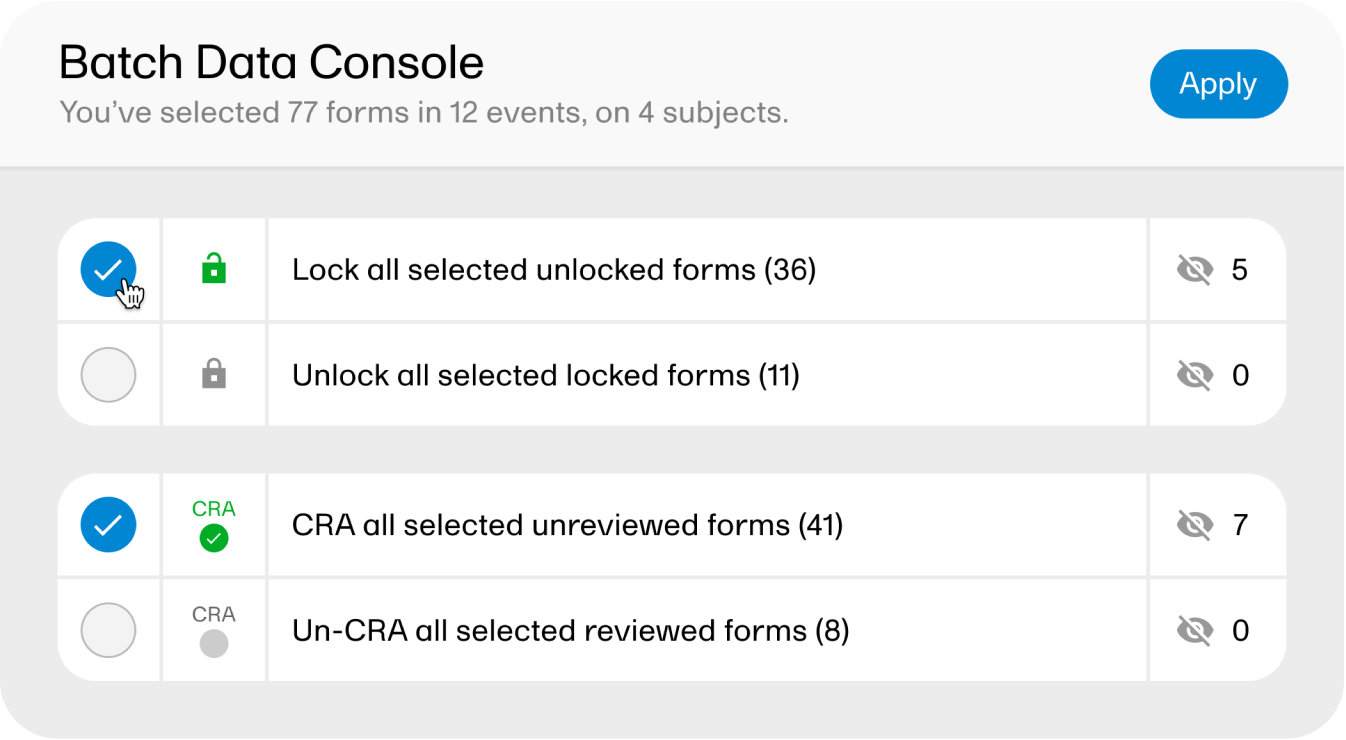
Switching on batch mode lets you handle activities such as data review, data lock, or data signing for multiple patients and events, all in one go.
Yes, statements of compliance to various regulations, including 21 CFR Part 11, as well as data privacy laws, can be provided upon request.
Instead of comparing us with other vendors, we let the system speak for itself. Anyone with interest can try out Viedoc free of charge for a limited time by simply sending us an email. We also let our clients share their experiences with us, and some statements are provided on this website.
Yes, Viedoc stores all versions of a form at the time the form is saved, and these are all under the control of the investigator.
Backups are taken, encrypted, and replicated to the paired Microsoft Azure region every five minutes. One full backup for each instance is encrypted and transferred to cold storage in a third location, read back, restored, and tested for integrity every 24 hours.

"One of the best things about Viedoc EDC is that it is very user-friendly and easy to use. One can navigate around Viedoc with relative ease. Also, customer support provides replies within 1 working day."
— Richard M.

"Viedoc is a very user-friendly platform. In fact, I prefer it over other competitors. Easy to navigate through and has very good features. The user training provided is quite simple to understand."
— G2 verified user

"Data capturing has been made easy because of viedoc, it is incredibly robust and easy to navigate"