
Here are six reasons why Viedoc is the right fit for you.
Viedoc Clinic can be configured to enforce data validation rules and checks, so you can ensure that the data entered into the system is accurate and complete. You can also set different permissions so that only certain roles have access to certain types of data and patient information.
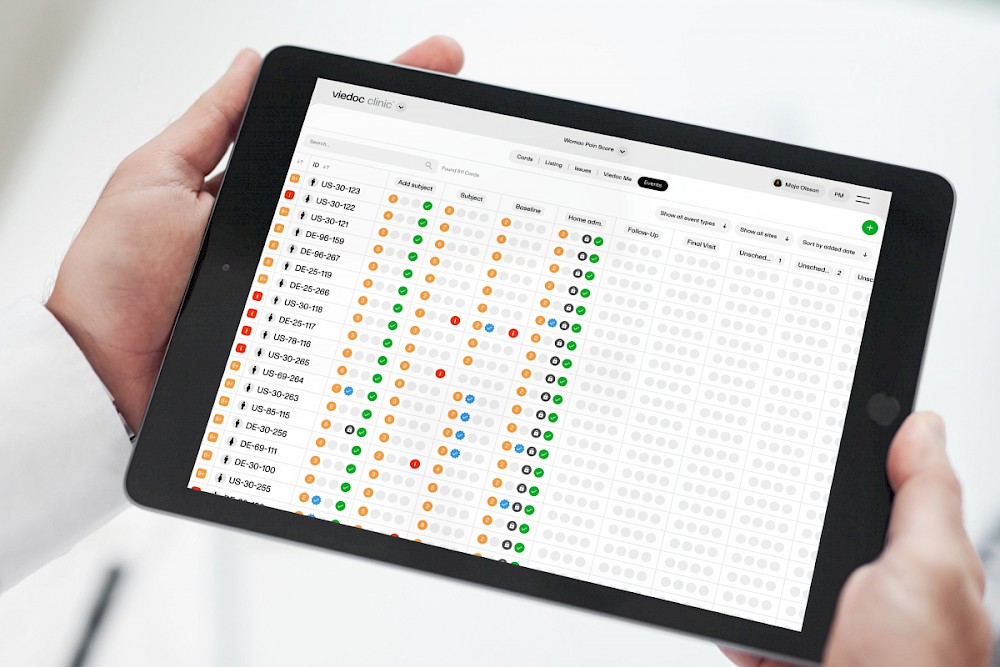
The Viedoc system can streamline many tedious and time-consuming tasks associated with data management, such as data entry and report generation. With Viedoc Reports, you can download and generate detailed reports from the patient to site to the country level, saving you time and effort.


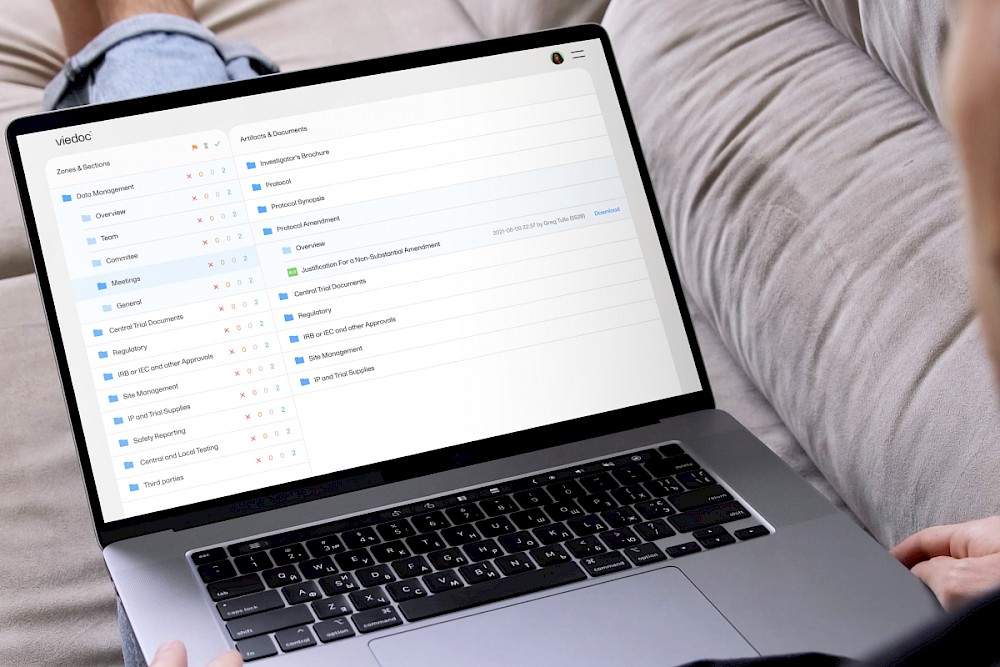
Viedoc TMF is used to organize and store data in a structured and easily searchable format, which can make it easier to find and analyze the data you need. The software sources the essential files in your studies, so you can more easily satisfy regulators and audits.
Viedoc TMF supports the global standard, DIAs Reference Model, that provides a standardized way to organize the documents under zones, sections, and artifacts. That way, you can find articles faster.
Viedoc is cloud based. This means you can access it online anywhere at any time. Since users have access whenever, wherever, and can share data with team members and collaborators, regardless of their location, they can strengthen and improve collaboration and communication.
Viedoc can be configured to protect data with security measures such as two-factor authentication and user access controls, which can help prevent unauthorized access to the data. As well, it is compliant with GDPR, HIPPA, APPI, and GB/T 35273-2020. Viedoc as a company also implements an ISMS and is certified according to ISO 27001.


The Viedoc solution meets the requirements of regulatory bodies, such as the FDA, and helps organizations stay compliant with relevant regulations. With Viedoc your files are always a few clicks away, saving you time when it comes to inspections.
At Viedoc, we have world-class professional services that will help you with any questions or concerns every step of the way.
If you want to save time, effort, and costs, while boosting your security while centralizing your workflow, you can book a demo with our support team.

"Viedoc's UI always amazes me. Clean, simple, convenient and user friendly."